- 24/7 Free Consultation: (888) 424-5757 Tap Here To Call Us
Chicago Depo-Provera Lawsuit

Facing health complications after using a medication such as Depo-Provera, an injectable contraceptive, can be incredibly overwhelming and intimidating. It’s not only about your physical well-being. There often are emotional, financial, and legal stressors to deal with all at once. At times like these, support from experts who understand your situation is essential.
You don’t have to navigate this situation alone; Rosenfeld Injury Lawyers is here to help. Our legal team understands that what you need most right now is someone who listens, takes action, and aggressively and compassionately works toward achieving justice on your behalf.
Depo-Provera Lawsuit Updates
If you’re worried about an increased risk of meningioma due to your medical history of taking Depo-Provera, it’s important to be aware of what’s going on with lawsuits that have been filed against its manufacturers. Below is an overview of how these cases are progressing.
2025
February 22
A Lawndale woman has filed a new Depo-Provera lawsuit in the U.S. District Court for the Central District of California, claiming that Depo-Provera caused her to develop an intracranial meningioma. She began using Depo-Provera in 2011 and says she was never told about any potential risks.
Over time, she developed serious symptoms like severe headaches, dizziness, vision and hearing loss, and seizures, which led doctors to discover a meningioma in 2015. She had to get a craniotomy and then receive six months of radiation, resulting in serious physical and financial challenges.
The suit, filed in California, is now headed to the Depo-Provera MDL in Florida.
February 21
The Depo-Provera cases had their first hearing on this date. The U.S. District Judge overseeing the Depo-Provera brain tumor lawsuits has identified five initial “pilot” cases to go to trial first in the MDL. These cases will move forward at an accelerated pace to gauge how future claims may unfold.
Additionally, on March 3, 2025, the court will hold an in-person Rule 26 conference, and the Joint Rule 26 Report must be submitted by March 7, 2025. A follow-up case management conference is set for March 10, 2025, where the court will review the report and discuss the next steps. In addition, anyone proposing a Plaintiffs’ lawyers leadership slate must submit their plans by February 28, 2025, so the judge can make decisions on appointments.
February 17
Judge Rodgers has released Pretrial Order No. 4 to oversee all Depo-Provera lawsuits in a unified way. A new master docket is now in place, allowing attorneys to file documents that either apply to all cases or just one.
The order also relaxes rules for out-of-state lawyers, letting them skip the usual Certificate of Good Standing and avoid double-paying fees if they paid them in earlier Depo-Provera lawsuits. Florida attorneys need to alert the court when they appear, and service of legal documents must follow federal guidelines unless both parties agree otherwise. This streamlined process is meant to efficiently handle the large number of cases likely to arise.
February 10
Recent estimates suggest that 16,651 to 30,261 meningioma cases may be tied to using Depo-Provera among women between the ages of 18 and 55. Case numbers could be even higher because some diagnoses are probably delayed.
February 7
The U.S. Judicial Panel on Multidistrict Litigation consolidated 27 Depo-Provera lawsuits into MDL No. 3140 in the Northern District of Florida, overseen by Judge M. Casey Rodgers, known for handling the $6 billion 3M earplug settlement.
These cases allege that prolonged use of Depo-Provera increases the risk of intracranial meningiomas, with claims that the manufacturers failed to warn consumers. In addition to the centralized lawsuits, 41 related cases are pending across 15 districts, making this an evolving and significant litigation.
What the Florida Venue for the Depo-Provera MDL Means for Plaintiffs
Selecting the right venue can play a major role in how a lawsuit progresses. Different courts have varied rules, distinct jury demographics, and specific methods for tackling large-scale cases. Here’s what plaintiffs should know about the Florida MDL:
Faster Timeline – Judge Rodgers, known from the 3M litigation, tends to move cases along quickly and isn’t open to stalling tactics.
Potentially Tougher Jury Pool – Northern Florida may not be as business-friendly as New York, but it’s also less plaintiff-friendly than California, meaning jurors may be more skeptical about awarding large sums.
Streamlined Discovery – Judge Rodgers generally keeps mass tort proceedings on track, which prevents Pfizer from stretching out the process. Her approach helps maintain momentum, reducing the risk that plaintiffs become discouraged and settle for less than they deserve.
February 1
Waiting for a decision on the location for the Depo-Provera class action lawsuit. A decision from the JPML (Judicial Panel on Multidistrict Litigation) is expected soon. If the Depo-Provera MDL is not in California federal court, state courts might become more involved in this Depo-Provera litigation.
January 30
A JPML hearing was held to determine where the Depo-Provera brain tumor lawsuits will be housed. Plaintiffs argued that creating a single, centralized MDL would make the process fairer for everyone involved. They named the Northern District of California, the Western District of Pennsylvania (as an alternative), the Northern District of Florida (before Hon. M. Casey Rodgers), and the District of New Jersey.
They withdrew their bid for the Central District of California because the recent fires posed problems for travel and scheduling.
Defense counsel, appearing on behalf of Pfizer, Prasco, Greenstone, and Viatris, also agreed on the need for a Depo-Provera MDL to streamline the cases. However, they pushed for the Southern District of New York, likely because it’s a major jurisdiction with experience in handling large, complex litigation.
The panel overseeing the hearing asked only a few questions and did not show a clear preference for any district. Both sides expressed the importance of creating this MDL, though they had different views on the best location. It’s now up to the panel to decide which court will become home to this coordinated effort.
Why Both Sides Agree an MDL is Appropriate
Both sides agree that creating an MDL is the most practical way to handle the growing number of Depo-Provera cases. The benefits of establishing an MDL include:
- Consolidating discovery procedures and sharing information
- Joint motion hearings that lead to more uniform rulings
- Plaintiffs coordinating legal arguments while preserving individual claims
- Shared litigation costs
- The possibility of a comprehensive settlement that benefits all affected parties
Some of the key points the MDL is likely to focus on include:
- Determining whether ingredients in Depo-Provera shots are directly linked to meningioma brain tumors
- Assessing whether Pfizer failed to warn U.S. users about tumor risks while including warnings on European and Canadian labels
- Investigating whether the defendants concealed known safety concerns in their dealings with another pharmaceutical company, Viatris
- Examining if the low-dose Depo-SubQ version could have lowered the likelihood of brain tumors in certain users.
January 29
Attorneys are filing the most compelling lawsuits first, prioritizing cases with severe injuries, long-term Depo-Provera use, and clearly documented medical histories.
January 25
A recent study revealed that medroxyprogesterone acetate (MPA), the main ingredient in Depo-Provera birth control, substantially increases the likelihood of developing meningiomas. The researchers reviewed records spanning from 2006 to 2020 and found that women who used medroxyprogesterone acetate for more than a year were 3.55 times more likely to be diagnosed with meningiomas compared to those who took a common oral contraceptive.
This risk stayed high even among women who continued medroxyprogesterone acetate for several years, with factors like obesity, past radiation exposure, and earlier contraceptive use accounted for.
Meningiomas make up around 40% of all brain tumors, raising serious worries about the long-term effects of medroxyprogesterone acetate. The European Medicines Agency (EMA) has recommended adding an adequate warning to the drug’s label, but so far, the U.S. Food and Drug Administration has not followed suit. This gap leaves many questioning whether American patients fully understand the potential risks linked to the use of Depo-Provera.
January 17
A new class action lawsuit was filed to push for the establishment of a medical monitoring program for women who used Depo-Provera for over a year and are at risk of developing brain tumors. The plaintiffs argue that women who have been using Depo-Provera should be closely monitored by healthcare professionals to detect any early signs of tumors, which could lead to better outcomes through early intervention.
This class action seeks to ensure that women who have used the drug are provided with the necessary medical surveillance and care to mitigate the long-term health risks associated with its use.
January 18
A 2024 study by David Bailey strengthens causation claims in Depo-Provera brain tumor lawsuits, showing that stopping progesterone-based drugs like Depo-Provera or other types of hormone replacement therapy can lead to the regression of meningiomas. This supports both general and specific causation in court. The findings highlight the potential for these tumors to diminish once the hormonal stimulus is removed. Such evidence could play a significant role in shaping legal strategies and medical decisions for individuals impacted by these cases.
January 12
A Woburn woman has filed a new Depo-Provera lawsuit in the District of Massachusetts, naming Pfizer, Viatris, Greenstone, and Prasco as defendants. She claims prolonged use of Depo-Provera from 1996 to 2005 led to an intracranial meningioma. Although there’s a motion to set up an MDL, many attorneys believe it might be better to wait until next month to file cases outside of California and New York. That way, they could potentially benefit from a more efficient process once the MDL coordination is finalized.
January 10
The U.S. Judicial Panel on Multidistrict Litigation (JPML) will meet in Miami to decide if the growing Depo-Provera lawsuits should be grouped under MDL No. 314. Pfizer agrees that consolidating the lawsuits is necessary to manage the volume of claims, although the central question is whether they’ll proceed in New York or California.
This isn’t a class action lawsuit; it’s an MDL, which allows cases to be combined for pretrial work under one judge without taking away each plaintiff’s individual claim. Grouping them in this way helps handle the wide range of injuries, including brain tumors, linked to Depo-Provera.
January 3
A newly filed lawsuit in San Diego alleges that Pfizer, along with other named parties, failed to properly warn a patient about Depo-Provera’s possible health risks, resulting in a meningioma after years of use. The plaintiff contends that Pfizer either knew or should have known about the drug’s dangers but chose not to update U.S. warnings, even though changes were made internationally.
She seeks both compensatory and punitive damages, claiming negligence, inadequate warnings, and a flawed product design. The lawsuit argues that a proper disclosure of risks might have prevented or lessened the harm she experienced.
2024
December 30
Pfizer says it has a defense based on federal preemption because the FDA supposedly turned down its request to include a meningioma warning on the Depo-Provera label. However, Pfizer hasn’t yet shared the actual approval request or any communication with the FDA. During discovery, more should come out about Pfizer’s label change effort and interactions with the agency.
Meanwhile, the plaintiffs argue that they have a strong design defect case, highlighting Pfizer’s other product, Depo-SubQ 104 Provera, which uses a lower dose and is equally effective. They also point out that the design defect claim isn’t affected by the Supreme Court’s Wyeth v. Levine (2009) ruling.
In that case, the Court held that compliance with Food and Drug Administration (FDA) labeling requirements does not shield a drug manufacturer from state-level failure-to-warn claims. This decision established that drug manufacturers have an ongoing responsibility to update their warning labels to reflect new risks or information, even after a drug has been approved by the FDA.
The ruling clarified that federal law does not necessarily override or preempt state law claims regarding inadequate labeling, thereby allowing patients to pursue legal remedies for injuries caused by insufficient warning or labeling.
December 27
Pfizer and other defendants agree to MDL for Depo-Provera lawsuits, but the debate continues over whether it should be held in New York or California. California arguably makes more sense due to the number of Depo-Provera lawsuits filed there.
December 23
Pfizer claims that the FDA blocked updates to Depo-Provera’s label regarding meningioma risks, setting up a preemption defense.
December 21
A new Depo-Provera lawsuit has been filed in the Eastern District of Louisiana by a woman who says Depo-Provera caused her to develop an intracranial meningioma. She claims no one warned her about this increased risk, even though research, along with updated labels in Europe and Canada, addressed these concerns.
The lawsuit also alleges that the product’s makers failed to alert U.S. users or share information about safer, lower-dose alternatives. This filing comes as the Judicial Panel on Multidistrict Litigation considers consolidating related cases in Northern California, an option many see as the most suitable location for these lawsuits.
December 18
The JPML will hear oral arguments on consolidating Depo-Provera lawsuits into an MDL, with a decision expected in early February.
December 1
A newly filed case in California asserts that a woman’s extensive use of Depo-Provera led to the development of two meningiomas in her skull, which required major brain surgery. The plaintiff accuses Pfizer and other defendants of knowing about the link between this Depo-Provera and meningiomas yet withholding this information from U.S. patients and healthcare providers, despite warnings issued in Europe and Canada.
She received 76 injections over 19 years and continues to experience vision problems along with other ongoing effects. The lawsuit requests both compensatory and punitive damages based on allegations of negligence and failure to warn, referencing 1980s studies that connected progestin-based medication to meningiomas.
November 26
Lawyers for 22 women have filed federal lawsuits against six companies behind Depo-Provera and its generic versions, alleging the contraceptive is linked to brain tumors. They are seeking to consolidate these lawsuits in Northern California into an MDL against Pfizer, Pharmacia & Upjohn Co. LLC, Greenstone LLC, Viatris Inc., Pharmacia LLC, and Prasco LLC.
November 25
The American College of Obstetricians and Gynecologists (ACOG) has responded to research showing Depo-Provera users may face a 5.5-fold higher chance of getting meningiomas than non-users.
They played down this finding by saying the risk amounts to “five out of 10,000 women,” which can overlook the alarming jump in risk. Additionally, ACOG called meningiomas “benign,” not fully recognizing the life-altering physical, emotional, and financial toll these brain tumors can cause.
November 22
A new Depo-Provera lawsuit filed in California named not only the drug manufacturers but also medical groups and pharmacies as defendants. The plaintiff, Madison Le, says these organizations – including Kaiser Permanente International, Kaiser Foundation Health Plan Inc., and The Permanente Medical Group Inc. – knew about the birth control’s dangers. According to the lawsuit, they continued promoting and profiting from Depo-Provera, even after conducting their own research that reportedly confirmed the risks.
November 14
Tina Stephens-Smith and her husband, Harold Albert Smith III, are the first plaintiffs to file a new Depo-Provera lawsuit against Pfizer in Nevada, marking the third state involved in this litigation.
The complaint alleges that prolonged use of the contraceptive Depo-Provera led the plaintiff to develop multiple meningiomas in her skull, ultimately resulting in invasive surgeries and ongoing health concerns. She used Depo-Provera for more than 20 years and claims that Pfizer, Viatris, Greenstone, Prasco, and other companies were negligent in failing to alert her or the medical field to the birth control’s connection to potential brain tumors.
In 2023, doctors identified several meningiomas – including one substantial tumor behind her eye – that required a craniotomy followed by radiation therapy. Despite these measures, other tumors have not shrunk enough, creating the need for additional surgeries and constant medical monitoring.
With increasing numbers of lawsuits targeting Depo-Provera’s manufacturers, many are now focusing on the Central District of California as a potential venue for the class action lawsuit MDL.
November 12
Pfizer has included a warning about the meningioma risk in Depo-Provera’s Canadian product monograph since 2016 but has not provided such an adequate warning for U.S. users. This is leading to Depo shot lawsuits from women in the U.S. who were not warned about the risks.
November 11
A woman from Cypress, California, and her husband have filed a claim, alleging that years of Depo-Provera caused her to develop multiple meningiomas. She began receiving Depo-Provera in 2015 and underwent about 36 treatments over nearly a decade.
In 2024, she started noticing symptoms like blurred vision and was subsequently diagnosed with two brain tumors in vital areas, prompting ongoing medical care. This lawsuit, which mirrors similar complaints about extended Depo-Provera use, was filed in federal court in the Central District of California.
November 4
Mayra Valencia filed a lawsuit in California, alleging that Depo-Provera caused her to develop a meningioma.
The complaint claims the drug’s manufacturers knew or should have known about the risk, referencing Canadian warnings since 2015 and studies linking progesterone to meningioma dating back to 1983. Valencia was receiving Depo-Provera injections for 23 years, leading to a craniotomy to remove the tumor.
October 31
Pfizer updated the Depo-Provera label in the European Union and United Kingdom to include a caution about meningiomas tied to prolonged use. The change recommends careful prescribing for patients with a history of these tumors and suggests stopping use only after diagnosis. The warning states:
“Meningioma: Meningiomas have been reported following long-term administration of progestogens, including medroxyprogesterone acetate. Depo-Provera should be discontinued if a meningioma is diagnosed. Caution is advised when recommending Depo-Provera to patients with a history of meningioma.”
Plaintiffs’ lawyers argue that this approach doesn’t address risks for people with no prior meningioma history and fails to inform them of the condition’s seriousness.
They point out that even if a similar warning is added in the U.S., it may still leave many patients uncertain about the potential dangers.
October 30
A strict liability design defect claim is central to the litigation. According to these Depo-Provera lawsuits, the company never sufficiently warned consumers about the link between the drug and meningiomas. Beyond the standard failure-to-warn angle, plaintiffs accuse Pfizer of a design defect tied to the drug’s potent dose of progestin, saying it was inherently harmful despite proper manufacturing practices.
They argue that Pfizer intentionally held back a safer option, Depo-SubQ Provera 104, in order to keep its market share. As a result, many people used the higher-dose version without realizing they could have significant health risks. The fact that Pfizer already had a safer, lower-dose product but did not promote it underlines the strength of the design defect claim.
October 29
The number of active Depo-Provera lawsuits is currently too small for a typical class action lawsuit but sufficient to potentially establish an MDL, with a growing number of claims expected.
October 28
A new Depo-Provera lawsuit was filed in California by Kathleen Fazio against Pfizer, Viatris, Inc., Greenstone, LLC, Prasco Labs, Pharmacia, and Upjohn. Plaintiffs plan to ask the Judicial Panel on Multidistrict Litigation to consolidate the cases. Attorneys are steering clear of New York due to previous unfavorable rulings and are instead filing in states like California, Massachusetts, Vermont, and Illinois, which recognize innovator liability for generic Depo-Provera claims.
Innovator liability is a legal doctrine that holds brand-name medication manufacturers liable for harm caused by generic versions of their drugs, even if they did not produce or sell the generic product in question. This principle is based on the argument that brand-name manufacturers are responsible for the initial design, development, and labeling of the drug, which generic manufacturers subsequently duplicate.
October 24
A lawsuit related to Depo-Provera was filed in federal court after concerns emerged about significant health risks, including loss of bone density and, later, brain tumors. The case alleged that Pfizer neglected to adequately warn users about these risks.
October 21
The lack of a meningioma warning in the U.S. label is a significant issue, especially given the warnings in Europe. Plaintiffs argue that Pfizer should be held accountable for this oversight.
October 17
Lesley Noble and her husband, Justin, filed the first Depo-Provera brain tumor lawsuit outside of California in Indiana federal court. Noble used Depo-Provera for more than 23 years, experiencing side effects like dizziness, fatigue, and incontinence. She had surgery in July 2017 to remove a large meningioma but continued using Depo-Provera for over a year, eventually requiring radiation therapy to stop the tumor from growing again.
The Nobles argue that Pfizer failed to properly investigate and warn consumers about the possible connection between Depo-Provera’s hormones and meningioma, noting that research suggesting such a link surfaced as early as 1989.
October 15
Meningiomas are often labeled as “benign,” but plaintiffs argue that this misrepresentation minimizes the severe impact these tumors have on women’s health, including the risk of progression and the need for ongoing treatment from healthcare providers.
October 6
There has been a wave of Depo-Provera lawsuits into Philadelphia’s Court of Common Pleas, which is known for managing heavy mass tort and product liability dockets. Plaintiffs from other states are filing because Viatris Inc. is based in Pennsylvania, allowing the court to exercise jurisdiction.
Pennsylvania’s more flexible procedures and rules on expert testimony also make it an appealing venue. Philadelphia juries have a track record of holding large corporations accountable, which could encourage defendants like Pfizer to negotiate fair Depo-Provera settlements.
At the same time, these lawsuits may arise in both a federal multidistrict litigation (MDL) context and as smaller, coordinated cases in state courts around the country.
October 1
Kristina Schmidt filed a lawsuit in California’s federal court against Pfizer Inc. and additional defendants, marking the first legal action linking Depo-Provera to an elevated risk of brain tumors. Schmidt accuses Pfizer and Depo-Provera’s former owners of long-standing knowledge – or at least having a responsibility to know – about the contraceptive’s increased potential for causing meningioma, and failing to properly warn consumers.
September 30
Depo-Provera’s link to meningiomas might arise from its synthetic progestin, which stimulates tumor growth in women with prolonged exposure. Projected Depo shot lawsuits for Depo-Provera contraceptive injections grow as studies show a 5.5-fold increase in the risk of meningiomas, leading to an increase in Depo-Provera lawsuits filed.
September 25
A Depo Provera MDL is the most likely route for the Depo-Provera litigation rather than a class action lawsuit, due to the need for individual evaluations of damages and the different nature of the injuries suffered.
September 22
According to plaintiffs’ lawyers, Pfizer’s strong influence over authorized generic makers (including Greenstone, Viatris, and Prasco) justifies holding the company responsible for not warning patients about Depo-Provera’s meningioma risks. They allege that Pfizer used its ownership and operational control to oversee how these generic products were marketed.
As a result, Pfizer’s failure to update safety labels falls under their responsibility for both the original contraceptive and its authorized generics. The argument here is that by maintaining such direct involvement, Pfizer had a duty to ensure accurate risk information was provided to consumers.
August 7
A recent article in the Journal of Neuroimaging encourages thorough imaging before surgery for patients who may have meningiomas linked to Depo-Provera. The authors spotlight new imaging methods aimed at improving surgical plans and care for people dealing with these tumors. They emphasize that a more in-depth approach can help doctors tailor treatment, particularly for women who face brain tumors tied to Depo-Provera.
July 12
Despite mounting evidence, Pfizer has not yet updated the warning label for Depo-Provera injections, which is a critical issue in the Depo-Provera litigation.
On April 25
Pfizer released a statement about the newly identified link between Depo-Provera and meningioma brain tumors, which was highlighted in a BMJ study. The company acknowledged the risk that prolonged use can pose and said it’s working with regulators to update the product’s labeling and patient information.
March 27 A study published in the British Medical Journal linking Depo-Provera to meningiomas significantly strengthens the legal cases, with women taking Depo-Provera being over 5 times more likely to develop these brain tumors. With 74 million women worldwide having used Depo-Provera, further research is still needed to learn why it might increase tumor growth, and the potential legal implications are expected to be significant.
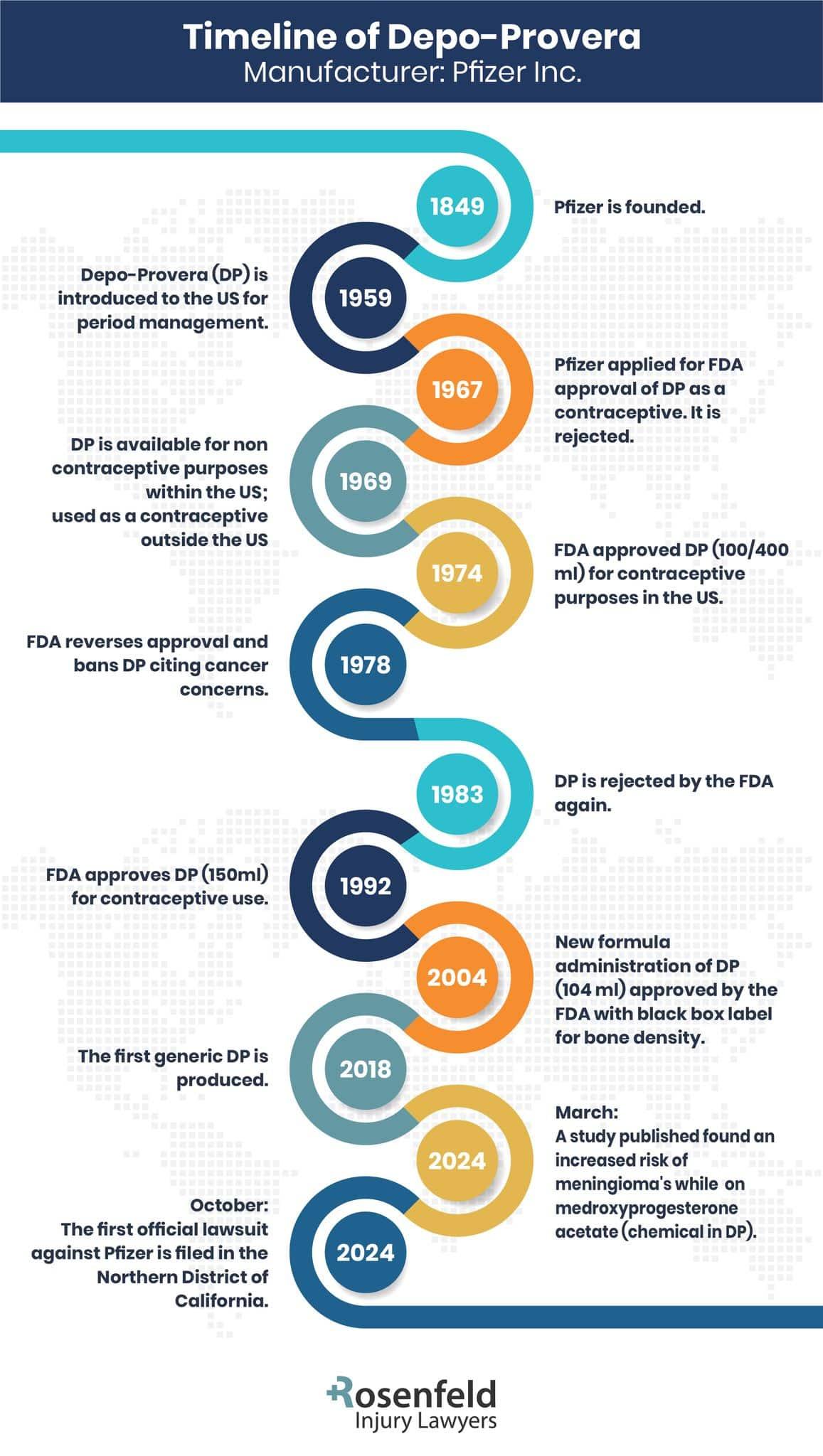
What Is the Depo-Provera Lawsuit About?
The Depo-Provera lawsuit focuses on the safety of this widely used hormonal contraceptive injection, particularly its potential connection to the development of brain tumors. The active hormone in Depo-Provera, medroxyprogesterone acetate, has been at the center of concerns following research suggesting that prolonged use of the injection may lead to the formation of brain tumors, including intracranial meningiomas.
These findings have prompted lawsuits alleging that Pfizer, the company behind Depo-Provera, failed to adequately warn patients about the health risks associated with the contraceptive. Women bringing these legal claims argue that they were not properly informed about the possible dangers of using Depo-Provera, specifically the increased risk of developing brain tumors. Many plaintiffs state they would have considered alternative options or avoided receiving Depo-Provera injections altogether had they been fully aware of the risks.
The growing number of cases reflects the serious impact this issue has had on women and their families. Some have experienced significant health challenges, with treatments and surgeries disrupting their lives. Others speak out about the emotional toll of living with a brain tumor diagnosis they believe could have been prevented. By filing these lawsuits, affected women are seeking accountability from Pfizer and striving to raise awareness for other potential victims.
For those who’ve been affected, it’s not just about financial compensation – it’s about securing justice and ensuring that pharmaceutical companies are held responsible when patients are harmed. The process may seem overwhelming, but there is help available for those who choose to seek justice.
Pfizer’s Earlier Knowledge of Major Health Risks
Evidence from studies published in the 1980s and 1990s paints a concerning picture of prior knowledge regarding the risks of Depo-Provera. Research during this period began to report unexpected findings of meningioma growth in patients who had been receiving Depo-Provera injections for extended periods.
By 1985, reports in scientific journals noted the potential for progestin in Depo-Provera to influence the growth of hormone-sensitive tumors, including meningiomas. Internal reviews and regulatory conversations also suggested that Pfizer, then Upjohn, may have been aware of the connection early on.
Despite these findings, the company continued to market the product without updating warnings in the U.S., even as labeling changes were applied in Europe and Canada.
The Dose-Response Relationship: One of the most concerning aspects of the link between Depo-Provera and meningiomas is the clear dose-response relationship. The risk of developing meningiomas increases with prolonged use of the drug, meaning the longer a person is on Depo-Provera, the greater their chances of being affected.
This relationship has been demonstrated in multiple studies, including the BMJ study. The dose-dependent nature of the risk further emphasizes the need for informed decision-making and transparency with patients who use Depo-Provera for extended durations.
Tumor Regression After Stopping Depo-Provera: Another notable discovery in the scientific exploration of this issue is tumor regression when using Depo-Provera is discontinued. Studies have shown that when patients cease use of the drug, meningiomas can shrink in size, which strongly supports the causal relationship between Depo-Provera and tumor growth.
This regression points to the direct role of synthetic progestins in stimulating tumor development. While encouraging, the potential for regression does not eliminate the physical and emotional stress or the risks associated with undergoing surgery or managing a preexisting tumor.
Why U.S. Patients Weren’t Warned Despite International Updates
Despite mounting evidence and action in other countries like Canada and those in Europe, U.S. patients were left in the dark. Regulatory decisions in other regions added warnings about the risks associated with prolonged use of Depo-Provera by the 1990s.
However, the same changes were not made for the U.S. market. This discrepancy highlights systemic gaps in how risks are communicated across different counties and raises serious questions about the prioritization of patient safety versus corporate interests.
Why Benign Meningiomas Are Still Dangerous
While the term “benign” may initially sound reassuring, it can be misleading – it does not mean the condition is harmless. Benign meningiomas are non-cancerous, but their growth can still have devastating effects on a patient’s health and quality of life. These tumors grow slowly but steadily, and depending on their location in the brain, they can press against critical structures. This pressure may lead to symptoms such as persistent and severe headaches, vision loss, difficulty with coordination, or even personality changes.
Neurological Risks Based on Size and Location
The impact of a benign meningioma on an individual’s health is closely tied to both its size and its location within the brain. These tumors, while non-cancerous, can exert significant pressure on surrounding brain tissue, cranial nerves, and other vital structures, leading to a variety of neurological complications. Below are some considerations regarding the risks based on size and location:
- Location-Dependent Risks
Tumors located near the cranial nerves, particularly those responsible for sensory input, may result in hearing loss, facial pain, or numbness.
Meningiomas pressing against the optic nerve can lead to vision impairment or, in severe cases, blindness.
If situated near areas governing motor functions, such as the motor cortex, the tumor can compromise movement, coordination, or muscle strength, potentially limiting mobility or causing paralysis.
- How Size Plays a Role
Small tumors might remain asymptomatic for an extended period; however, as they grow, symptoms such as severe headaches and cognitive difficulties may emerge.
Larger tumors create greater pressure on the brain, increasing the likelihood of seizures, which could become chronic and require long-term management.
Enlargement of a meningioma can obstruct cerebrospinal fluid flow, resulting in hydrocephalus (fluid buildup in the brain), which may require urgent medical intervention.
- Progression and Severity
While most benign meningiomas grow slowly, persistent growth eventually impacts critical brain functions, leading to temporary or permanent disabilities depending on the areas affected.
The Challenges of Surgical Treatment
When it comes to larger meningiomas, surgery is often the only viable treatment option. However, brain surgery is inherently complex and involves significant risks. Below are some of the primary challenges associated with the surgical treatment of meningiomas:
- Risk of Complications
Surgical procedures on the brain carry the potential for serious complications, including infection, excessive bleeding, swelling, or even stroke. The location and size of the tumor significantly influence the likelihood of these risks.
- Incomplete Resection
For tumors located in sensitive or difficult-to-reach areas, complete removal may not be possible without compromising critical brain functions. This can necessitate additional surgeries or adjunct therapies, such as radiation therapy, to reduce the size of the remaining tumor or control its growth.
- Extended Recovery Period
Recovery from brain surgery can be physically and emotionally demanding. Patients may require weeks or months to regain strength and function. The process may involve physical therapy and rehabilitation to address lingering issues such as cognitive impairments, speech difficulties, or reductions in mobility.
- Long-Term Complications
Depending on the extent of the surgery and the area of the brain affected, some patients may face permanent challenges. These include memory problems, personality changes, or speech and motor function deficits, all of which can significantly impact quality of life.
Given these challenges, treatment decisions must be highly individualized, taking into account the patient’s overall health, the tumor’s characteristics, and the potential risks versus benefits of surgical intervention.
The Fact That it’s Preventable Adds to the Burden.
For individuals affected by benign meningiomas that may be linked to preventable causes, the frustration and emotional impact can be even greater. Knowing that their condition could have been avoided often amplifies feelings of anger and helplessness. This underscores the importance of raising awareness about potential risks tied to medications or treatments that may contribute to the development of brain tumors.
Even though these tumors are labeled “benign,” the reality is that they can dramatically alter a person’s life. This is why legal action becomes an essential step for many.
Eligibility to File a Depo-Provera Brain Tumor Lawsuit
Potentially affected women must consider several important factors to determine eligibility to file a Depo-Provera lawsuit. These factors can significantly influence the course and outcome of the legal action. A thorough understanding of the following criteria will help clarify eligibility for a Depo-Provera lawsuit.
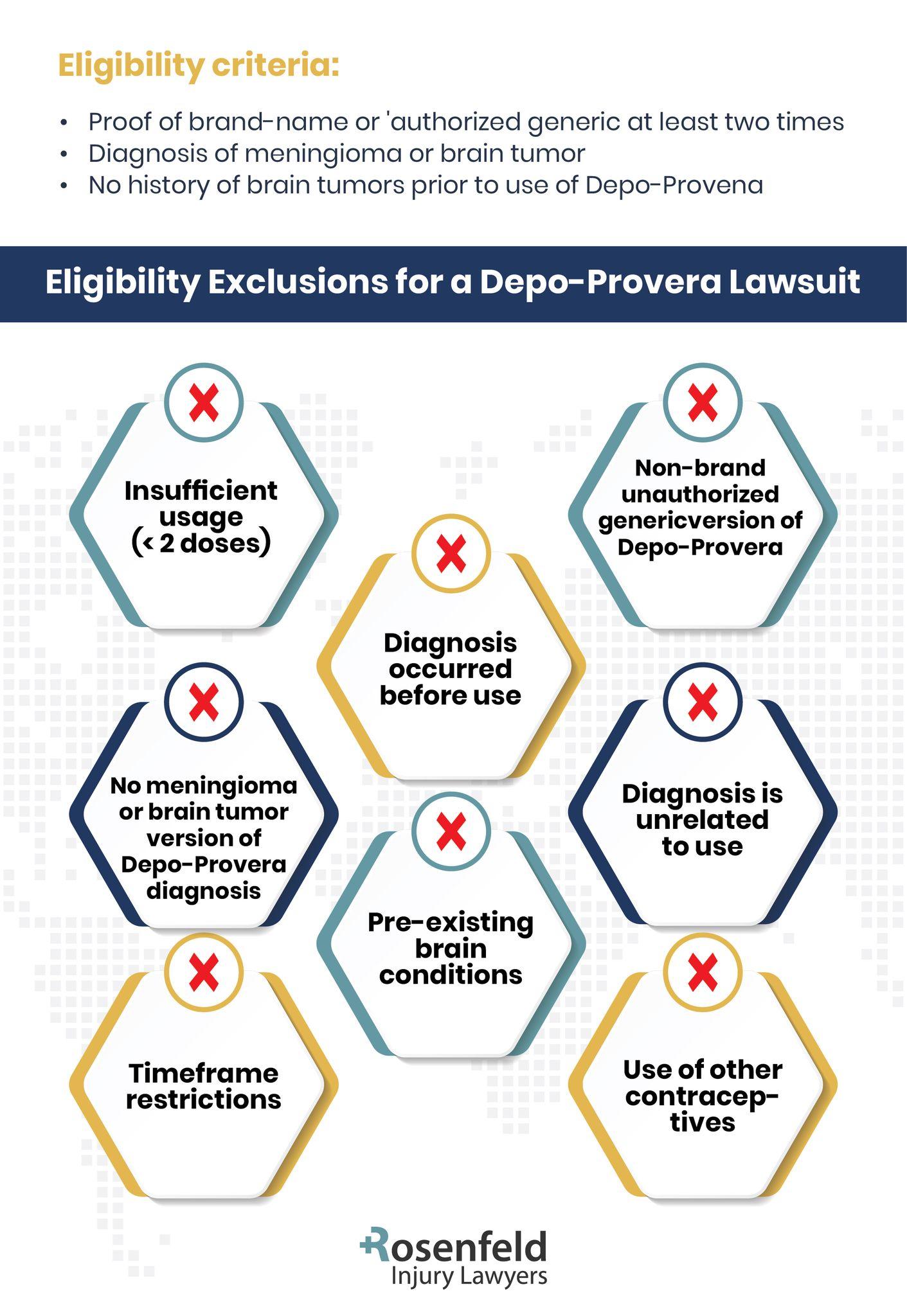
Use of Depo-Provera
Individuals who have received Depo-Provera injections – the contraceptive injection of medroxyprogesterone acetate – may have grounds to file a lawsuit if they have experienced serious health consequences.
Depo-Provera, used for birth control, has been linked to certain adverse effects, including the potential risk of developing brain tumors. For women who trusted this contraceptive method and later faced severe health challenges, such as meningiomas, the impact has been life-altering.
Development of Health Conditions
Eligibility for pursuing a Depo shot lawsuit generally hinges on whether you’ve suffered certain health conditions, such as brain tumors. Affected women who have developed meningiomas, which are often categorized as benign but can have severe consequences, may have a valid claim.
The development of these tumors has raised concerns about the long-term effects of taking Depo-Provera, especially for individuals who relied on its safety assurances. Legal claims can also extend to other serious medical conditions potentially tied to the injections, depending on the evidence provided by healthcare providers and medical records.
Statute of Limitations
The statute of limitations for filing a Depo-Provera lawsuit is an essential factor to consider, as it determines how long individuals have to take legal action after their diagnosis or discovery of a related health problem.
This time frame varies by state and can depend upon specific circumstances, such as when the link between the injection and the condition was identified. Acting quickly is important, as missing the statute of limitations could make it impossible to pursue compensation, regardless of the severity of the health impacts.
By consulting with an attorney familiar with Depo-Provera cases, individuals can gain clarity about the specific deadlines that apply to their situation.
Causal Link to Depo-Provera
Proving a causal link between Depo-Provera and the health condition in question is a vital component of these cases. This process typically involves presenting comprehensive medical records that detail the timeline of Depo-Provera administration and the eventual diagnosis of conditions such as meningioma brain tumors. These records must clearly document the frequency and duration of Depo-Provera use alongside relevant clinical findings.
Additional evidence, including imaging results such as MRIs or CT scans, pathology reports confirming the presence and characteristics of the tumor, and expert medical opinions, can provide support and strengthen the argument. Testimonies from medical professionals can also offer insight into the plausibility of a connection between the drug and the health condition.
Legal Assistance and Consultation
Seeking legal assistance is an important step for those affected by potential side effects of Depo-Provera. Experienced attorneys familiar with these cases can provide valuable guidance and support throughout the legal process. They help individuals understand their rights, assess the available evidence, and determine the next steps for pursuing compensation.
Law firms, such as Rosenfeld Injury Lawyers, offer free consultations to evaluate whether a case might qualify for legal action. These consultations can provide clarity about options for moving forward and answer questions about filing claims. With their knowledge of mass tort litigation, attorneys can help streamline what might otherwise feel like a very confusing and overwhelming process.
For many women and their families, understanding how the law applies to their circumstances can ease decision-making and provide a sense of direction. By working with attorneys who handle Depo-Provera lawsuits, individuals work with a team of professionals that can handle the legal complexities, allowing them to focus on recovery during this stressful period.
How to File a Depo-Provera Lawsuit
Filing a Depo-Provera lawsuit requires understanding the legal complexities and nuances associated with potential health risks related to Depo-Provera. Below is an overview of how to navigate these legal proceedings.
Case Evaluation
The first step in filing a lawsuit involves evaluating whether you have a valid case. Attorneys experienced in Depo-Provera claims will review your medical records, including your history of Depo-Provera use and any related diagnoses. They will also ask about your symptoms, medical treatments, and how this has affected your life.
This stage is not just about proving the link between the medication and your health issue but assessing how these complications have impacted your physical, emotional, and financial well-being.
During this phase, it’s important to gather as much documentation as possible. Medical records, prescriptions, and even doctor’s notes will help attorneys build a strong foundation for your case. Most attorneys will offer a free consultation to help you determine if you are able to move forward with a legal claim.
Consulting with Legal Professionals
Consulting with a lawyer is an important step if you believe you’ve been harmed by a medication. A lawyer who specializes in these cases can help you understand your rights and explain the legal process in straightforward terms. They’ll evaluate the details of your situation, including medical history and any supporting documentation you’ve collected.
Attorneys can also help identify any legal deadlines that may apply, ensuring you take action in time. Many specialize in handling cases like yours, so they’re familiar with the challenges involved.
A dangerous drug attorney can assist in deciphering how progesterone receptors and the possible risk of brain tumors factor into your case considerations. Reaching out to a lawyer doesn’t mean committing to a lawsuit immediately; it means getting information to make informed decisions about your next steps.
Filing an Individual Lawsuit
Once your case is evaluated, the next step is to file an individual lawsuit. This type of lawsuit is different from a class action case because it allows you to tell your story and present the specific damages you’ve experienced. Your case will typically be filed in a court that has jurisdiction in your area or where the drug manufacturer conducts business.
Many Depo-Provera lawsuits are later consolidated through a process known as Multidistrict Litigation (MDL). This approach is often used for cases with similar claims to improve efficiency during the pre-trial process. However, unlike class actions, MDL cases remain independent. Each plaintiff’s circumstances, experiences, and potential settlement or trial outcomes are considered on an individual basis.
Filing an individual lawsuit offers a path to seek compensation for the harm that Depo-Provera caused. While the legal process may seem complex, it is designed to give each person their own voice in court. An experienced attorney can help make the process more manageable and ensure that your case is presented effectively.
The MDL Process
When numerous individuals are harmed by the same product, as in the case of complications stemming from Depo-Provera, lawsuits may be consolidated into an MDL. This process is designed to make handling similar cases more efficient while ensuring fairness for all involved. Below is an overview of the MDL process so you have a better idea of what to expect.
- Centralizing Similar Cases: MDLs are created when many lawsuits share common questions of fact, such as whether a product was defective or if a company failed to warn consumers of potential risks. Instead of handling each lawsuit separately, they are temporarily consolidated in one court under one judge. This streamlines the process and avoids conflicting pretrial rulings in different jurisdictions.
- Pretrial Proceedings: During this stage, both parties – victims and the defendant – work through the discovery process. This involves gathering evidence, questioning witnesses, and sharing information related to the case. For example, documents from the manufacturer, such as internal research or communications, may be reviewed. Pretrial motions are also addressed during this phase, as each side refines its arguments and positions.
- Bellwether Trials: Bellwether trials are essentially test cases. A small number of cases are selected to go to trial to help both sides understand how juries might respond to the evidence. These outcomes don’t directly decide the remaining cases but can influence Depo-Provera settlement negotiations. Based on the results, parties may decide whether to resolve cases collectively or continue to pursue them individually.
As the MDL progresses, there is a possibility that the defendant (usually the manufacturer) may offer a Depo-Povera settlement.
What This Means for You
If you are part of an MDL, your case will benefit from the shared resources and focused legal oversight this process provides. Understanding how the MDL process works can help you feel more confident about your path forward. An attorney experienced in MDL cases will ensure your rights are protected and your voice is heard.
Settlement Negotiations
Many lawsuits, including those involving Depo-Provera, are resolved through settlements rather than going to trial. Settlement negotiations typically begin after the discovery phase, once both sides have gathered and reviewed evidence. During this process, your attorney will work with Pfizer or its representatives to reach a settlement that fairly compensates you for the harm you’ve experienced. This might include compensation for medical bills, lost wages, ongoing health needs, or the emotional and physical impact of your injuries.
Every settlement offer is unique, as it reflects the specific details of your case. If a settlement is presented, your attorney will explain the terms clearly, so you fully understand what accepting it would mean. It’s important to know that you are never required to agree to a settlement. If the offer doesn’t align with your needs or the damages you’ve experienced, you can decline.
Your attorney will help guide you through that decision and explain the next steps if further negotiations or a trial becomes necessary. This process ensures that your voice is heard and that any decision you make feels right for your individual situation.
Going to Trial
If a fair Depo-Provera settlement cannot be reached, your case may go to trial. Cases can often reach settlement even while a court case is underway, so just because your case is scheduled for trial doesn’t mean you won’t end up settling.
During the trial, your attorney will present evidence showing how Depo-Provera caused your complications and the resulting impact on your life. The court will hear from medical experts, review all relevant documents, and weigh the damages you’ve suffered. After this process, a judge or jury will determine if you are entitled to compensation and, if so, how much.
Trials can be lengthy and require patience, but they are sometimes necessary to achieve justice when settlement negotiations fall short.
Navigating a Depo lawsuit can seem overwhelming, but understanding the steps involved can make it more manageable. An experienced attorney will guide you through this process, answering your questions and advocating on your behalf every step of the way.
Who Are The Defendants In The Depo-Proveral Lawsuits?
Understanding the defendants in Depo-Provera lawsuits is crucial for developing an effective legal strategy. The primary parties being named in these cases include the following:
Pfizer Inc.
As the primary defendant, Pfizer Inc. holds significant responsibility due to its control over the New Drug Application (NDA) for Depo-Provera. Pfizer has overseen the drug’s production and labeling, making it a central figure in any related litigation.
With substantial financial resources, partly bolstered by recent profits from COVID-19 vaccines, Pfizer is seen as the main target defendant due to its proximity to decision-making processes related to the drug’s safety.
Greenstone, LLC
Greenstone, LLC, a subsidiary of Pfizer, operates as an “authorized generic” manufacturer and distributor of Depo-Provera. It produces chemically identical versions of the drug but without the branding typically associated with the name Pfizer.
Allegations against Greenstone include its role in enabling widespread distribution of the product without adequately differentiating it from its brand-name counterpart. This has led to claims that Greenstone contributed to a lack of transparency and created potential confusion among patients and healthcare providers.
Prasco Labs
Prasco Laboratories is another distributor that handles “authorized generics” of Depo-Provera. The allegations against this company focus on its marketing and labeling practices, which closely resemble those of the branded product.
Concerns have been raised about whether these practices misrepresented the drug or misled consumers about potential risks. Prasco’s involvement highlights issues with the way generic products are marketed and how this may have affected those who used this version of Depo-Provera.
Pharmacia & Upjohn
Pharmacia & Upjohn played an initial and important role in developing, submitting, and managing the NDA for Depo-Provera before Pfizer acquired the company. The allegations against Pharmacia & Upjohn center on its handling of safety concerns during the early stages of bringing the drug to market.
Some claims suggest the company may not have done enough to address or mitigate potential risks tied to Depo-Provera’s safety profile. Its actions remain relevant due to its part in the drug’s history and development.
Viatris Inc.
Emerging from the 2020 merger of Upjohn, Greenstone, and Mylan N.V., Viatris Inc. is implicated in these mass tort lawsuits for its role in distributing and selling both Depo-Provera and its “authorized generic” counterparts. The legal scrutiny of Viatris focuses on its participation in the wider distribution network of the drug in question.
The presence of multiple defendants in Depo-Provera lawsuits highlights the complexity of navigating pharmaceutical litigation and underscores the importance of identifying the role of each party in the distribution and safety oversight of the medication.
Pfizer’s Arguments and How They Can Be Refuted
One of Pfizer’s defenses in the failure-to-warn lawsuits involving Depo-Provera leans heavily on the legal concept of preemption. The company asserts that it made efforts to update the drug’s warning label to address concerns about potential side effects, but these changes were not approved by the Food and Drug Administration (FDA). Pfizer argues that because the FDA rejected their proposed updates, federal law takes precedence over any state-level requirements for additional warnings.
This defense hinges on the idea that Pfizer’s hands were tied by federal regulations, making it impossible for the company to satisfy both federal and state requirements at the same time. Essentially, they claim that they were unable to make changes to the label without FDA approval, and therefore they cannot be held liable under state law.
This argument has been used by many pharmaceutical companies in similar cases and often raises questions about how regulatory decisions impact consumer safety and the rights of individuals who experience harm.
While Pfizer’s position is built on the legal framework of preemption, it does little to address the experiences of patients who believe they were not adequately warned about the risks associated with Depo-Provera. This ongoing tension between federal oversight and individual accountability continues to shape the direction of these lawsuits.
Why Courts Generally Reject Preemption in Failure-to-Warn Cases
Despite Pfizer’s argument, courts in similar failure-to-warn cases often reject preemption defenses. The core principle is that pharmaceutical companies retain an independent responsibility to warn healthcare providers and patients of any known risks, even if the FDA fails to mandate or approve specific label changes.
The legal precedent suggests that companies cannot simply rely on FDA restrictions as a blanket defense to avoid accountability. Jurisdictions across the U.S. have emphasized the importance of proactive measures when patient safety is at stake.
Evidence That Undermines Pfizer’s Argument
Pfizer’s own actions cast doubt on the reliability of its preemption defense. While the company argues that it couldn’t update the Depo-Provera warning label in the United States, it updated the label in Europe to include additional risk information. This raises a troubling question – if Pfizer had the means to enhance the label abroad, why didn’t it do the same for patients in the U.S.?
The decision to strengthen warnings in one region but not another suggests that Pfizer had the ability to act but chose a different approach depending on the market. By leaving U.S. patients with less comprehensive warnings, despite evidence of harm tied to Depo-Provera, Pfizer’s inconsistent actions place their stated defense into question. Victims deserve transparency, and the lack of uniformity in warning practices undermines trust and accountability.
Voluntary Warnings Were Always an Option
Even if the FDA rejected certain label modifications, Pfizer still had the ability – and certainly the responsibility – to issue voluntary and adequate warnings to healthcare providers and patients. Pharmaceutical companies frequently use other methods to communicate safety concerns, such as “Dear Doctor” letters to physicians and public safety notices.
These steps would ensure people are informed about potential risks, even when formal label changes face regulatory hurdles. By failing to pursue these options, Pfizer’s claim that it did everything possible to warn consumers comes under significant scrutiny.
What This Means for Victims
The weaknesses in Pfizer’s preemption defense provide an opportunity for victims of Depo-Provera-related complications to challenge the company’s claims. Understanding that courts are unlikely to favor preemption in Pfizer’s failure-to-warn cases reinforces the importance of pursuing legal action.
Victims should consult experienced legal professionals to ensure their voices are heard and to seek appropriate compensation for the harm they have endured.
Why State Courts (Especially Philadelphia) Matter in Depo-Provera Litigation
State courts, particularly those in Philadelphia, are shaping up to be a significant battleground for litigation related to Depo-Provera. For Viatris, one of the primary defendants in these lawsuits, Pennsylvania holds particular importance since the company is headquartered there. This connection often makes Pennsylvania courts a natural choice for plaintiffs looking to pursue legal action.
It’s not just about that, though. Philadelphia courts have earned a reputation for being more favorable to individuals bringing cases against large corporations, especially in matters involving product liability or consumer safety. This has led to outcomes that often push companies to consider larger settlements or adjust their legal strategies.
The influence of Philadelphia courts goes beyond just the possible outcomes of individual cases. Decisions made in these cases can ripple outward, shaping how similar lawsuits are approached in other jurisdictions.
For victims of Depo-Provera who feel they were not adequately warned about its risks, taking their claims to state court in Pennsylvania could present a meaningful opportunity to have their voices heard in a legal setting where corporate accountability has often been a central focus.
This potential impact makes state court proceedings a vital part of the larger landscape of Depo-Provera litigation, offering a parallel path to the federal MDL process in pursuing justice.
Where Else Are Lawsuits Being Filed?
While federal MDL cases are centralized, many related lawsuits are also being filed in state courts across the country. Pennsylvania, especially Philadelphia, has emerged as an important venue for these cases due to its connection to Viatris. Victims are choosing to file in state courts when possible, knowing these courts might approach their cases differently than a federal MDL.
Beyond Pennsylvania, numerous lawsuits are being filed in other state courts across the United States. Jurisdictions such as California, New York, and Illinois have also become popular venues. These states are often chosen because of their reputation for handling complex Depo-Provera litigation and their ability to provide thorough judicial review.
By understanding where lawsuits are being filed and why Pennsylvania, and particularly Philadelphia, stands out, victims can better understand their options.
Filing Lawsuits After Using Generic Version of Depo-Provera Injections
Filing a lawsuit after using a generic version of Depo-Provera may seem confusing, but it’s important to understand how accountability works in these cases. Many people assume that lawsuits can only be brought against Pfizer if they used the brand-name version of the drug.
This isn’t entirely accurate. Pfizer can still be held responsible for some generic versions of Depo-Provera, specifically those sold by authorized distributors like Greenstone, Prasco, and Viatris. These authorized generics are not typical generics – they are produced and sold under Pfizer’s direct oversight.
Because Pfizer stays closely involved in the manufacturing and distribution of these authorized generics, the law often allows for the possibility of holding them accountable for injuries caused by these products. Women who have used an authorized generic version of Depo-Provera and experienced severe side effects, such as the development of brain tumors, should know they are not necessarily excluded from pursuing legal action.
Why Generic Depo-Provera Users Are Sometimes Confused About Lawsuit Eligibility
The distinction between generic drugs and authorized generics is often unclear, leaving many victims unsure about who may be legally responsible for their injuries. Some people assume that all generic drugs fall under different companies’ liability, making them ineligible to sue the original manufacturer. However, with authorized generics, Pfizer retains control, meaning their accountability extends beyond the brand-name drug.
If you believe the version of Depo-Provera you used has caused serious health problems, the first step is identifying whether it was an authorized generic overseen by Pfizer. This often requires reviewing your medical and pharmacy records, which should include details about the manufacturer or distributor of your medication. These records can provide important insight into whether Pfizer bears responsibility for the product you used.
To make sense of this information, consulting with a knowledgeable attorney is highly recommended. They can help review your records, confirm who produced the medication, and determine whether you have grounds for a lawsuit. Taking this step not only strengthens your case but also ensures you’re not navigating this complex process alone.
Understanding your rights can help you get the justice you deserve if your health has been harmed. Even if you didn’t use the brand-name version of Depo-Provera, you may still be eligible to hold Pfizer accountable if the product was one of their authorized generics.
Depo-SubQ Provera 104 – The Safer Alternative That Pfizer Ignored
Depo-SubQ Provera 104 was developed as an alternative to the original Depo-Provera contraceptive injection. This formulation aimed to address some concerns associated with the original by using a lower dose of medroxyprogesterone acetate, its active ingredient.
Additionally, this version is administered subcutaneously, or just beneath the skin, rather than intramuscularly, which can be a more invasive procedure. This method offered a promising way to maintain effective birth control while potentially reducing the side effects linked to the higher doses used in the original version.
Potential Benefits of a Lower-Dose Option
One of the most significant advantages of the Depo-SubQ version lies in the reduced exposure to medroxyprogesterone acetate, which some studies have linked to the development of meningiomas. By delivering a lower dose, this formulation could have offered a safer option for individuals concerned about long-term side effects like these. For many patients, the availability of a lower-dose alternative might have represented an opportunity to achieve balance between effective pregnancy prevention and minimizing health risks.
The less invasive subcutaneous administration method is also worth noting. Many patients find injections into the muscle to be more painful and intimidating. The ability to safely deliver the medication under the skin could have improved the overall experience for users and encouraged more consistent usage.
A Missed Opportunity for Broader Awareness
Despite the apparent advantages of Depo-SubQ Provera 104, there was a noticeable lack of awareness about this option among patients. Reports and anecdotal evidence suggest that many individuals who might have benefited from the lower-dose formulation were simply not informed that it even existed. This lack of promotion and visibility represents a serious problem, as it left countless people reliant on the higher-dose version without understanding the potential risks or alternatives.
Had Pfizer made a stronger effort to inform healthcare providers and patients, it’s possible that more individuals would have switched to this version of the Depo-Provera birth control. By doing so, they would have reduced their exposure to high levels of medroxyprogesterone acetate and the associated risks of conditions like meningiomas.
Financial Interests vs. Patient Safety
One likely explanation for the limited promotion of Depo-SubQ Provera 104 is the influence of financial priorities. Launching a newer contraceptive option may not have aligned with Pfizer’s larger business focus, particularly if it meant potentially cannibalizing profits from the original Depo-Provera product. This kind of decision-making raises significant concerns about whether patient safety was placed second to corporate interests – which it often is with these large companies.
The Importance of Transparency
Patients deserved comprehensive information to make informed decisions about their health. Greater transparency about the benefits and differences of Depo-SubQ Provera 104 might have helped some individuals avoid risks associated with higher-dose injections, particularly those already at increased risk for meningiomas.
The limited awareness of this version of the medication represents a failure to prioritize patient well-being. For those who have experienced complications, learning about the existence of a safer alternative adds to their frustration. Victims have every right to demand accountability from Pfizer for decisions that may have compromised their health.
Depo-Provera Lawsuit Settlements
Depo-Provera lawsuit settlements may range from $200,000 to $500,000 – though sometimes it may increase to even more than a million dollars – contingent on the severity of the diagnosed meningiomas.
The key determinant of settlement value is the Depo-Provera brain tumor’s grade, with Grade III (malignant) cases involving life-threatening circumstances potentially generating significantly higher compensation compared to Grade I (non-cancerous) meningiomas.
Grade III Meningioma Cases: For Grade III meningioma cases, the anticipated compensation could exceed $1 million, reflecting the grave medical outcomes associated with these diagnoses. Depending on how the Depo-Provera litigation proceeds, trial settlements could escalate to tens of millions, particularly if the court recognizes that there is substantial evidence of causation presented.
Pfizer’s Financial Stability: Pfizer’s financial stability has a significant bearing on the likelihood of large Depo-Provera settlements. Over the past few years, Pfizer has achieved substantial profits, including from its Covid-related products, putting the company in a strong economic position. This financial capacity means that Pfizer has the resources to handle large settlement payouts without threatening its overall fiscal health.
Because of this, women impacted by Depo-Provera are likely to have access to meaningful compensation for their claims, allowing them to address medical expenses, lost wages, and the emotional turmoil Depo-Provera caused.
Fear of the Diagnosis: For many, hearing they have a brain tumor sparks an overwhelming sense of fear. Even though meningiomas are often benign, the thought of something growing within the brain can be deeply unsettling. Patients may worry about how the tumor will affect their ability to think, work, or interact with loved ones. This fear can linger throughout the treatment process, adding to the emotional burden.
Anxiety About Surgery and Outcomes: The potential need for surgery or other medical interventions often creates severe anxiety. Patients may focus on the risks associated with the procedure or question whether their treatment will be successful. Even after surgery, concerns about the tumor returning can weigh heavily on the mind. This ongoing uncertainty can take a toll, impacting everyday decision-making and mental well-being.
The Psychological Toll of Living with a Meningioma: Beyond fear and anxiety, the experience of living with a meningioma can lead to deeper psychological struggles. Depression is not uncommon, as patients struggle with changes to their health or independence. Some may develop post-traumatic stress disorder due to the stress of undergoing invasive procedures or the sudden life changes that follow a diagnosis. Recognizing this emotional distress is an important step toward healing, as ignoring them may hinder recovery.
Coping with These Emotional Challenges
Understanding the psychological impact is just as important as dealing with the physical aspects of a meningioma. Seeking support from therapists, psychiatrists, or support groups can offer relief and provide tools to manage fear and anxiety. Including close friends and family in the conversation may also create a strong emotional support network for recovery.
There’s no way to know for sure how much you could be entitled to for a brain tumor-related Depo-Provera settlement ahead of a case review. Don’t let the physical and emotional toll stop you from seeking the justice you deserve.
Book a free case evaluation.
Statute of Limitations for Filing Depo-Provera Lawsuits
When considering legal action in relation to Depo-Provera, many women worry about the time limits for filing a lawsuit. The statute of limitations for Depo-Provera lawsuits may vary depending on the state where the case is filed.
In general, the time limit ranges from 1 to 6 years from the date of injury. However, there are two primary reasons that the statute of limitations may not be as big of an issue as it often is in other types of injury cases.
The Discovery Rule
The discovery rule is an important legal concept that can help women affected by Depo-Provera-related brain tumors pursue justice even years after using the drug. This rule allows the statute of limitations – the time limit to file a lawsuit – to begin not when the harm occurred, but when the individual became aware, or reasonably should have become aware, of the connection between their health conditions and the drug.
For those affected by Depo-Provera, the link to brain tumors like meningiomas only became clearer through more recent scientific studies. Before this research, many women may not have had the information needed to understand the link between their symptoms and the medication they had been prescribed. This lack of awareness often left them without the ability to take legal action within the original, standard timeframe.
The discovery rule gives these individuals an opportunity to seek accountability, even when the harm Depo-Provera caused was not immediately apparent. It acknowledges the time it can take for medical research to uncover such links and for patients to fully understand their diagnoses and their causes. For women living with long-term health impacts from Depo-Provera, this rule can make all the difference in giving them a fair chance to act.
Fraudulent Concealment
Fraudulent concealment occurs when a party intentionally hides or misrepresents information to prevent another from discovering important facts. This concept is particularly relevant in Depo-Provera lawsuits as well, as it is alleged that the manufacturer knew about potential links between the contraceptive and serious health conditions, like brain tumors, but failed to adequately warn patients and healthcare providers.
When a company withholds such information or misleads the public about possible increased risks of certain conditions, it can prevent individuals from recognizing the injuries Depo-Provera caused.
For many affected women, the lack of transparency may have delayed their understanding of the link between Depo-Provera and their medical conditions. Fraudulent concealment can pause the statute of limitations, allowing victims additional time to pursue legal action. This legal principle acknowledges that people cannot be expected to act on information deliberately hidden from them.
It ensures that companies are held accountable for unfair practices and gives victims the chance to seek justice, even if their injuries were discovered years after using the product.
Legal Precedents Favoring Plaintiffs
There have been several notable legal precedents where courts ruled in favor of plaintiffs in cases involving undisclosed drug risks.
Vioxx
One example is the litigation surrounding Vioxx, a pain reliever that was found to cause an increased risk of heart attacks. Plaintiffs in these cases successfully argued that the manufacturer, Merck, failed to provide adequate warnings about the potential dangers of the drug. Courts acknowledged the evidence showing that Merck knew about the increased risks but did not take appropriate steps to inform consumers. This set an important precedent for holding pharmaceutical companies accountable for withholding essential safety information.
Asbestos Exposure
Similarly, in lawsuits tied to asbestos exposure, plaintiffs have made substantial progress by demonstrating that manufacturers knew about the harmful effects of their products but chose to hide that knowledge.
These cases often highlighted internal documents and testimony that supported claims of fraudulent concealment by the companies. The outcomes of these lawsuits have paved the way for others in similar situations to pursue justice, especially when corporations prioritize profits over safety.
These precedents demonstrate that victims have legal pathways to seek justice when evidence shows companies acted irresponsibly or withheld important information – even if it’s been a long time since exposure. By leveraging these past rulings, individuals harmed by Depo-Provera may find legal frameworks already in place to support their claims. Legal teams with experience in similar cases can use these examples to effectively argue for fair outcomes for their clients.
These factors mean that many women who developed brain tumors after using Depo-Provera still have the opportunity to file new Depo-Provera lawsuits. By consulting with an experienced attorney, victims can better understand how these legal exceptions may apply to their situation.
How Rosenfeld Injury Lawyers Can Help
Rosenfeld Injury Lawyers brings expertise and dedication to assist you with any Depo-Provera lawsuit filed. With an understanding of the law and the medical intricacies involved, we are ready to help you navigate the complexities of legal claims related to Depo-Provera brain tumors and other associated risks. Below is how we can support you.
Comprehensive Case Evaluation
We take the time to carefully review your individual case, looking for the connection between Depo-Provera and your health struggles. This includes examining how medroxyprogesterone acetate, the active ingredient in Depo-Provera, may have impacted your body over time. If you’ve experienced brain tumors or other severe side effects, we gather the details needed to build a clear picture of how the medication may have played a role. Your story and health history matter, and our goal is to bring those details to light to support your case.
Legal Advice and Representation
Understanding the legal process can feel overwhelming, and that’s where we step in. Our team explains each step in simple terms so you know what to expect. Whether your case involves a national MDL or claims in a state court, we’re here to stand by your side. We provide straightforward legal advice and representation to ensure your voice is heard and your rights are protected throughout the process.
Medical Support
Your health is at the center of everything we do. That’s why we work closely with healthcare providers to help gather the medical evidence needed to strengthen your case. Whether it’s obtaining records, diagnoses, or expert opinions, we focus on proving how Depo-Provera may have contributed to your health concerns.
This could include evidence related to brain tumors, treatments like radiation and surgeries, or other related care. We ensure your case is backed by credible, clear documentation.
Negotiating Settlements
We understand how health struggles linked to medications can upend your life. That’s why we make every effort to secure fair settlement offers that reflect the challenges you’ve faced related to this birth control medication. From the physical toll of health conditions like brain tumors or bone density loss to the emotional and financial impact, we fight for compensation that truly acknowledges what you’ve been through. While every case is unique, our aim is to help you find some relief in the form of a resolution that addresses your needs.
Pursuing Justice Through Depo-Provera Litigation
If a resolution cannot be reached outside of court, we are ready to take your case to trial. We approach litigation with care, making sure every piece of evidence is presented clearly and effectively.
No matter how complex or challenging a case may be, we are ready to present your story in a way that supports your fight for justice and fair compensation.
Our commitment is to provide comprehensive legal and medical support, ensuring that you receive the justice and compensation you deserve for the adverse effects experienced due to Depo-Provera.
What Is Depo-Provera?
Depo-Provera is an injectable form of birth control that uses the hormone progestin to prevent pregnancy. Depo-Provera works primarily by inhibiting ovulation and thickening cervical mucus to make it difficult for sperm to reach an egg.
Depo-Provera is commonly administered as a shot every three months, offering a convenient alternative for women seeking an effective contraceptive option without daily intervention.
However, with healthcare providers recommending Depo-Provera for so many women, it’s essential to understand its associated risks and the legal claims surrounding its use.
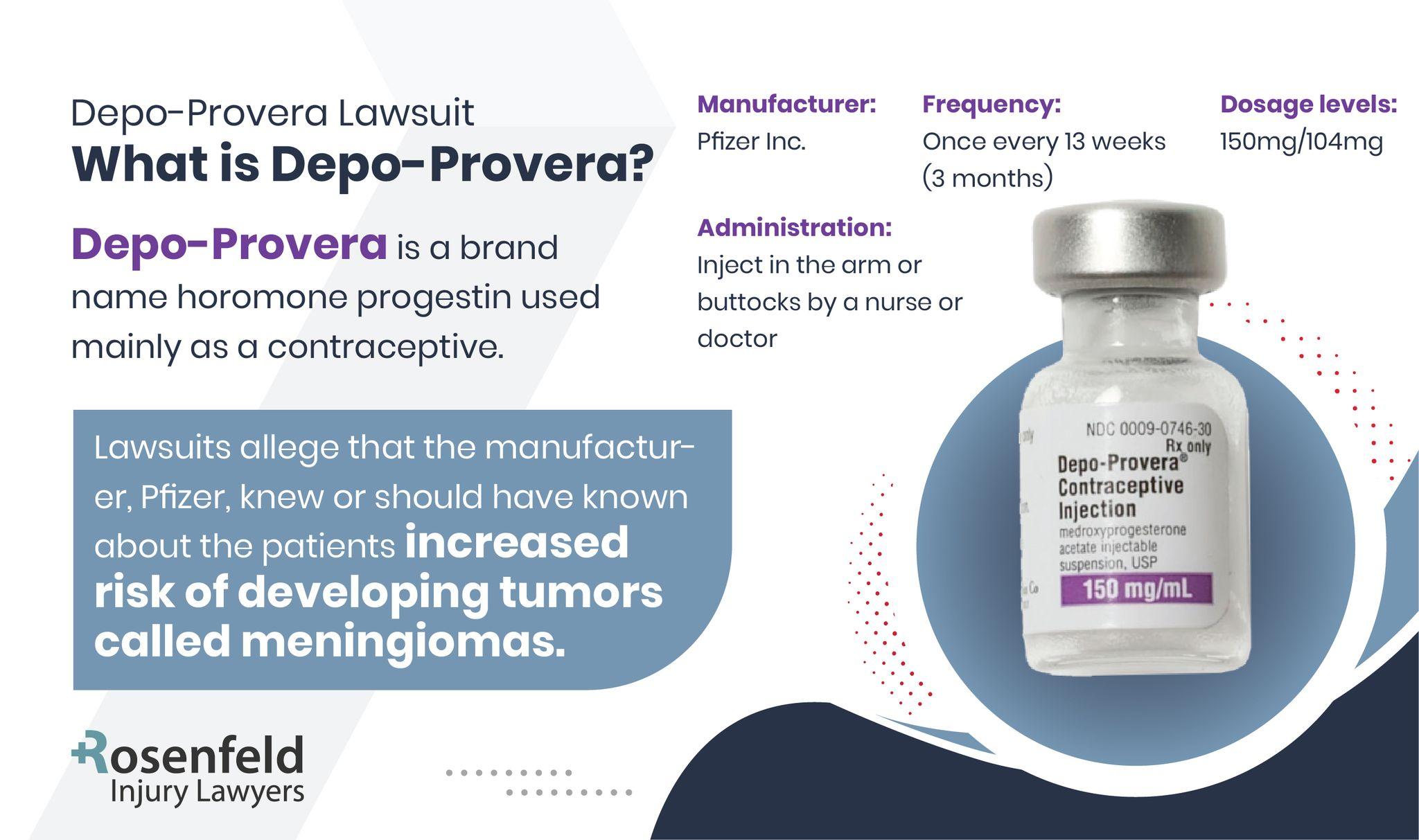
Depo SubQ Provera 104
An alternative formulation, Depo SubQ Provera 104, is available, which is administered under the skin rather than into the muscle, providing similar contraceptive benefits with potentially reduced side effects.
Both forms are prescription-based and should be discussed thoroughly with medical providers to weigh the benefits and risks, particularly considering the potential side effects of prolonged Depo-Provera use.
Potential Risks Associated With Depo-Provera Usage
Depo-Provera usage, while effective for contraception, carries several potential risks that need to be closely examined.
Meningioma Brain Tumors
Research has linked Depo-Provera use to brain tumors in some cases. These are typically benign growths arising from the meninges – the membranes that surround your brain and spinal cord.
Even though they are usually benign, women affected can experience issues with brain functions depending on the brain tumor size and location. This can potentially cause severe headaches and chronic headaches, vision problems, or neurological deficits. It can also lead to the need for serious medical interventions, like chemotherapy and surgery. Recognizing these symptoms early can be crucial for treatment and management.
Bone Mineral Density Loss
Another known side effect is the reduction in bone mineral density, which can make you more susceptible to osteoporosis or fractures later on. This occurs because the hormone progestin contained within Depo-Provera birth control can interfere with bone regeneration and strength.
Blood Clots
There’s also an increased risk for blood clots when using hormonal contraceptives like Depo-Provera, especially deep vein thrombosis (DVT) and pulmonary embolism (PE). Those clots can become life-threatening if they travel through your bloodstream to vital organs such as the lungs or heart.
Weight Gain and Mood Changes
As with many hormonal contraceptives, Depo-Provera users often experience side effects such as weight gain, mood changes, reduced sexual interest, and occasional severe depression.
History of Evidence of Depo-Provera’s Dangers
Over the years, a growing body of evidence has raised concerns about the potential dangers of Depo-Provera. These findings include risks such as meningiomas and other serious side effects that have prompted regulatory action in various countries.
October, 2024
Pfizer has updated the Depo-Provera label in the EU and UK, warning of the increased risk of meningiomas with long-term use of progestogens (including medroxyprogesterone acetate). If a meningioma brain tumor is diagnosed, the label advises stopping Depo-Provera and being cautious when prescribing it to patients with a history of meningiomas.
September 31, 2021
A class action lawsuit settlement in Canada related to Depo-Provera’s risks of bone density loss provides a parallel to the ongoing litigation regarding meningioma brain tumors in the United States.
May, 2008
The Superior Court of Quebec granted certification to a nationwide class action lawsuit against Pfizer regarding Depo-Provera, citing concerns about the drug’s safety and potential risks to Depo-Provera users.
November, 2004
The FDA required a “black box” warning on the label for Depo-Provera’s contraceptive injection, acknowledging the risk of significant and irreversible loss of bone density with prolonged use of Depo-Provera birth control.
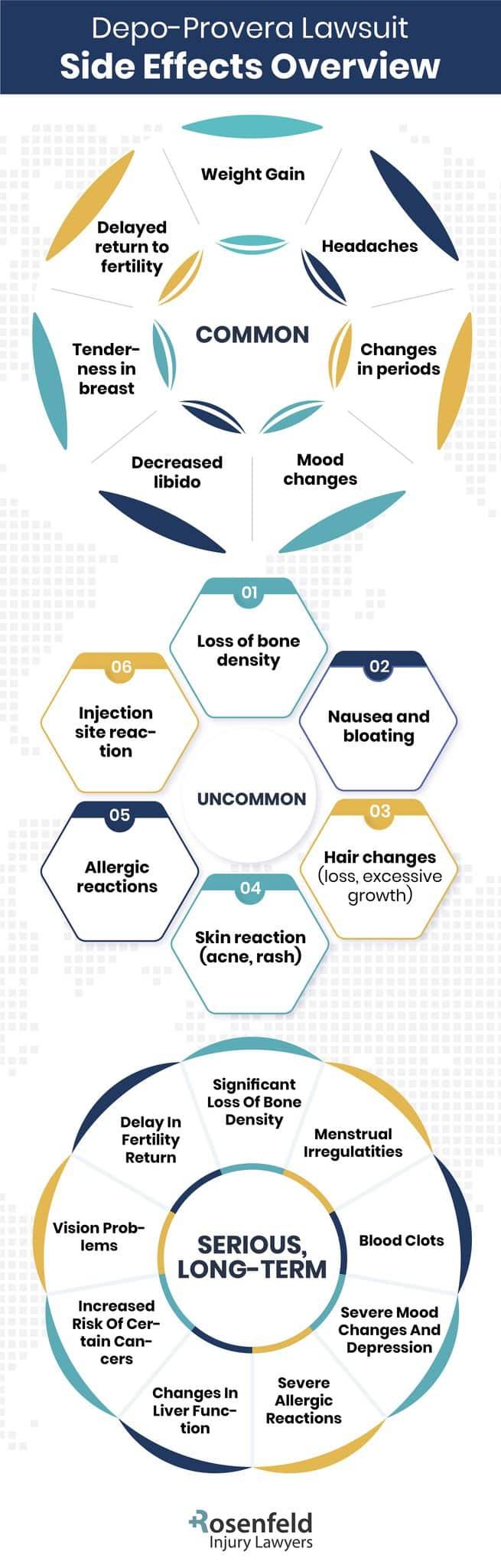
Legal Actions
The Depo shot lawsuit claims that the manufacturer, Pfizer, failed to adequately disclose these risks, which increased risks of brain tumors and other serious health complications for Depo-Provera users. The MDL concerning Depo-Provera is ongoing, with claims addressing both individual and federal court cases linked to severe health problems like brain tumors.
Understanding Depo-Provera’s implications is crucial so you can make informed decisions about birth control options. This information is vital for potential users and for those involved in a Depo shot lawsuit.
Is Depo-Provera Being Discontinued?
At the moment, Depo-Provera is not being discontinued. It remains a widely used form of long-acting reversible contraception (LARC) offered by medical providers for women seeking an alternative to daily oral contraceptives or other methods of birth control.
Consult a Depo-Provera Lawyer Today!

If you’ve experienced serious side effects after taking Depo-Provera birth control, consulting with an attorney who specializes in pharmaceutical litigation is one of the most important steps you can take. At Rosenfeld Injury Lawyers, we understand how difficult this situation can be, and we’re here to help you explore your legal options. Our experienced attorneys will review your case details and help you pursue a claim related to a brain tumor linked to Depo-Provera and other side effects.
Call (888) 424-5757 or complete our online contact form today.
Resources: [1] British Medical Journal
Related Posts:
Depo-Provera Linked to Brain Tumors
Depo Provera Lawsuit Settlement Values
All content undergoes thorough legal review by experienced attorneys, including Jonathan Rosenfeld. With 25 years of experience in personal injury law and over 100 years of combined legal expertise within our team, we ensure that every article is legally accurate, compliant, and reflects current legal standards.







